UC Berkeley’s Brilliant Breakthrough in Carbon Capture
A canary-yellow powder developed at UC Berkeley has jumped a long-standing carbon capture hurdle: it can absorb CO₂ from the air directly, and it can do so over and over without rapidly degrading.
This material, called COF-999, is the latest iteration of “covalent organic framework” technology developed by Omar Yaghi, professor of chemistry at UC Berkeley and senior author of the paper on COF-999 published in Nature in October. COFs are essentially tiny sponges: honeycombed tubes with a massive surface area and lots of holes that snag CO₂ molecules as air flows through. The challenge is that these porous frameworks are fragile, easily degraded by water, sulfur, nitrogen, and other airborne molecules.
UC Berkeley graduate student Zihui Zhou, the Nature paper’s lead author, spent years trying to create a stronger COF that could pull more carbon out of the air before degrading.
“I tried maybe hundreds of reactions, hundreds of materials,” says Zhou. “And after two years of work, we finally found this material.”
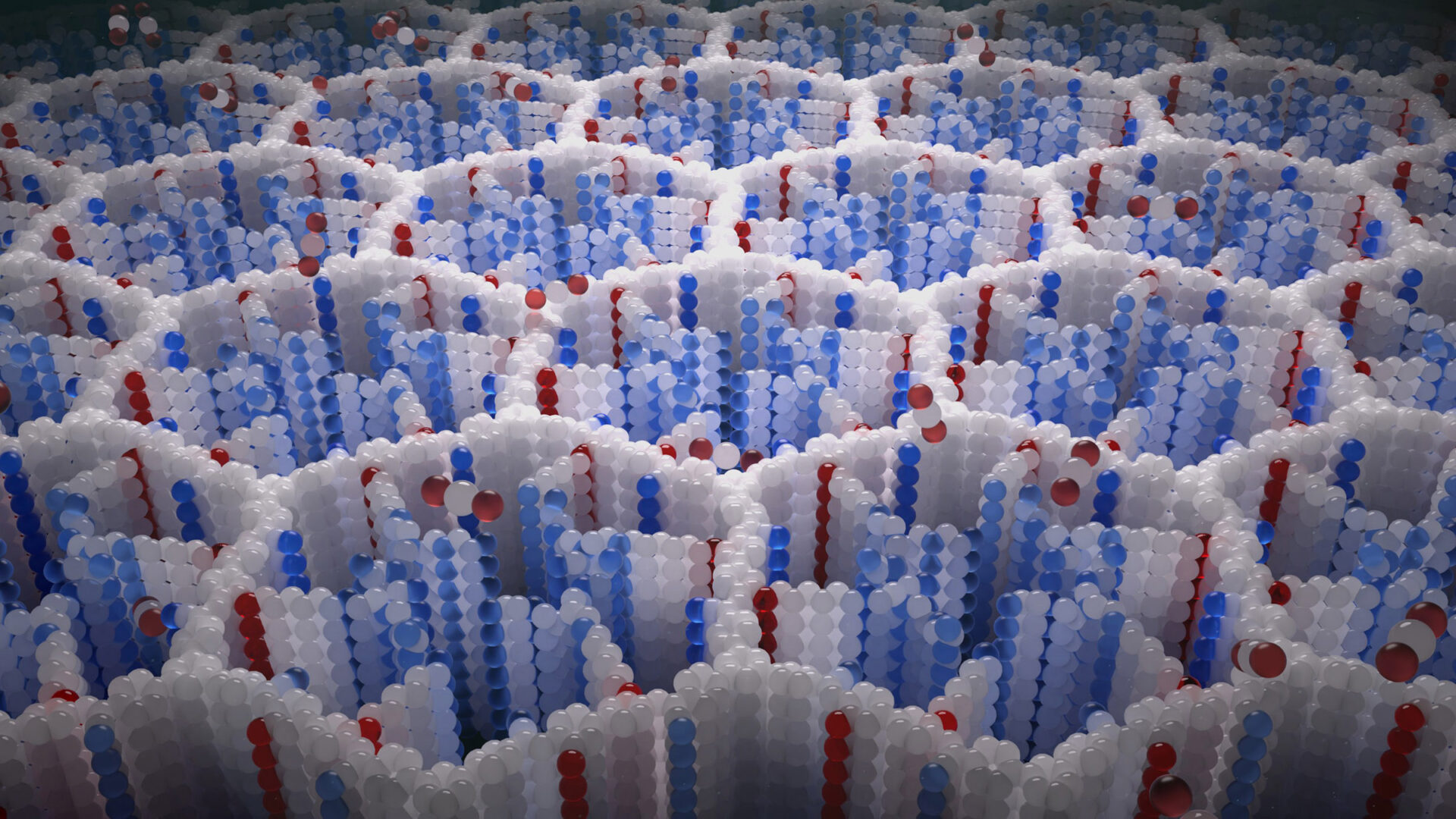
A visualization of COF-999’s crystalline structure. Image: Chaoyang Zhao
Zhou designed COF-999 to be stronger, stabler, and longer-lasting. The structure is studded with amines (NH2 groups), then flushed with more amines that form short amine polymers. As air passes through these structures, the CO₂ molecules bind to the amines. Unlike past direct air capture technologies, COF-999 actually works better in the presence of moisture.
“I think the exciting part was when I said to Zihui, ‘Why don’t we just pipe in Berkeley air and see if it takes up the CO₂?’” says Yaghi. “And it did – not just the excess CO₂, but all the CO₂.”
Then, Zhou carried out 100 catch-and-release cycles — scrubbing carbon from the air with COF-999, then heating it to release the carbon — without major efficiency loss. According to Zhou, less than half a pound of COF-999 can take up 44 lbs of CO₂ in a year — about as much as a tree.
While this new technology is exciting, it’s important to point out the limitations of Direct Air Capture. An International Energy Agency report estimates that, if all currently planned Direct Air Capture projects were to move forward, they could remove around 65 million tons of CO2 per year in 2030.
Other Recent Posts
Assistant Editor Job Announcement
Part time freelance job opening with Bay Area climate resilience magazine.
Training 18 New Community Leaders in a Resilience Hot Spot
A June 7 event minted 18 new community leaders now better-equipped to care for Suisun City and Fairfield through pollution, heat, smoke, and high water.
Mayor Pushes Suisun City To Do Better
Mayor Alma Hernandez has devoted herself to preparing her community for a warming world.
The Path to a Just Transition for Benicia’s Refinery Workers
As Valero prepares to shutter its Benicia oil refinery, 400 jobs hang in the balance. Can California ensure a just transition for fossil fuel workers?
Ecologist Finds Art in Restoring Levees
In Sacramento, an artist-ecologist brings California’s native species to life – through art, and through fish-friendly levee restoration.
New Metrics on Hybrid Gray-Green Levees
UC Santa Cruz research project investigates how horizontal “living levees” can cut flood risk.
Community Editor Job Announcement
Part time freelance job opening with Bay Area climate resilience magazine.
Global carbon emissions in 2024 were 37.41 billion metric tons. So, even at the best-case-scenario 2030 rate, it would take about 575 years to remove one year’s worth of emissions. Let’s imagine that our DAC capacity improves by 10,000%; it would still take about six years of capture to scrub 2024’s CO2 emissions. In short: if we’re still emitting significant amounts of new CO2 into the atmosphere, Direct Air Capture can do little to stop warming. But if advances like COF-999 are combined with driving down emissions and other carbon capture methods like reforestation, DAC could become a valuable piece of the decarbonization puzzle.
The next step for COF-999 is scaling up production, then actually deploying it. Yaghi anticipates relatively low costs — the material doesn’t require rare minerals or complex synthesis. “I estimate that, within a year, we would be able to make [metric] ton quantities,” says Yaghi.
Meanwhile, Zhou is working on improving the material further, pushing the COF’s efficiency by helping a higher percentage of the amines snag CO₂ during each cycle. It’s a slow, steady process of tiny tweaks and precise observation.
“Zihui probably makes hundreds of observations every day,” says Yaghi, proudly. “He has to make a decision about which observation is the most important to pursue. Much to his credit, he pursued the right observation.”
Yaghi isn’t new to making the news — but this is Zhou’s first major project with real-world implications. He was so excited about the data he was collecting that he texted Professor Yaghi about it almost every day, he says.
“The human aspect of this is often untold,” says Yaghi. “We leave the security of our home behind because we want to advance the frontiers of knowledge. And if you’ve worked hard and you have the right thinking, you will discover something that potentially could contribute to society. I think that this is how civilizations evolve.”